Yena Lee
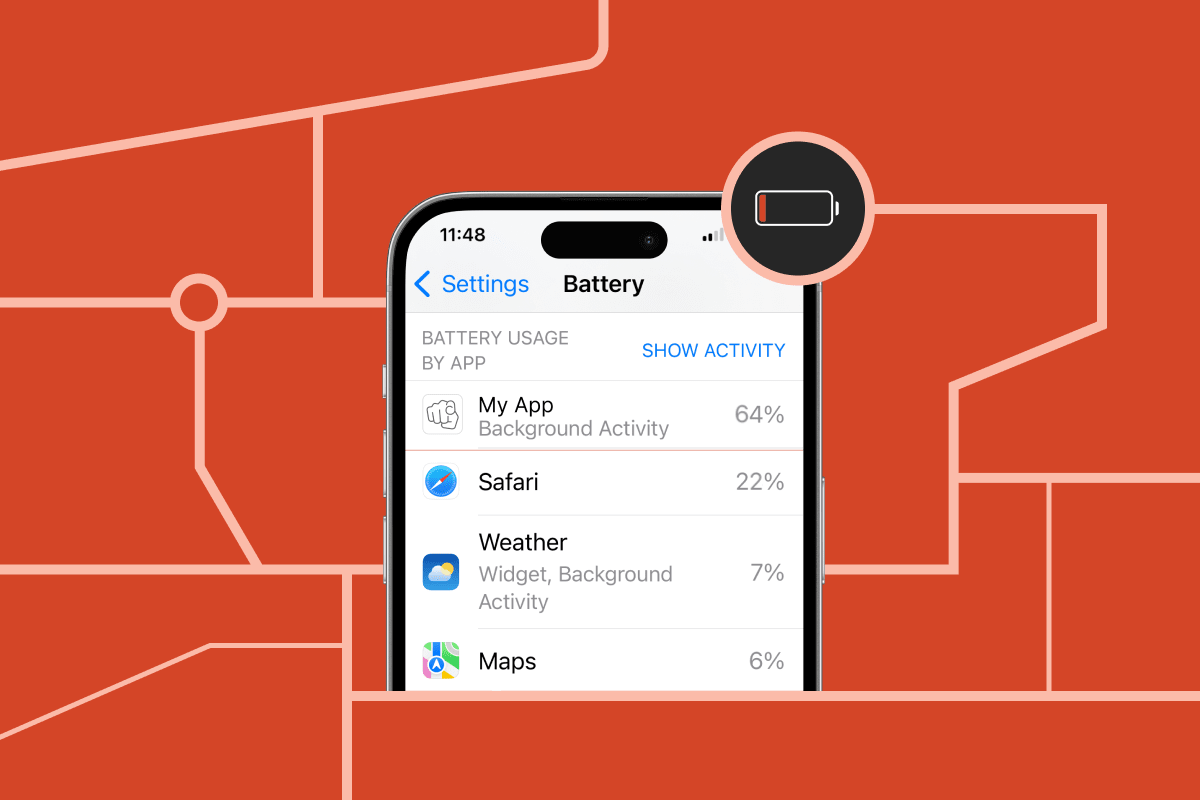
Lowering location accuracy is not the best way to reduce your app's power usage. Use these configurations for iOS background location services and stop draining your users' batteries.
Choosing a headless CMS can be a daunting task. We created a tool for you to chat with our headless platform expert Scott Fuller to help you find the best headless solution for your business.
Contentful, Sanity, and Strapi are the headless CMS platforms we recommend most often to our clients. Here's a comparison of features that matter, plus an in-depth guide to choosing the right headless CMS platform for you.

A headless CMS offers more flexibility to integrate with a variety of front-end solutions and devices, making it an excellent option for highly scalable and performance-focused websites. Non-technical team members can edit content, optimize for SEO and performance, and use the same content across different channels and touch points.

Plus, what AI won’t tell you about how to choose a headless CMS. Read how Sanity, Contentful, and Strapi compare on features that matter.

With design tokens, you can quickly and easily change the look and feel of websites and applications by simply updating values in one central location.
At our third Software as a Medical Device meetup, Danielle Dorfman Faruq (Regulatory & Quality Director at Kintsugi) shared learnings and tips on how to get your SaMD in the hands of real users faster, while meeting regulatory requirements.
Motion graphics are an effective way to add visual interest to any website or app. They're also a great way to highlight key points in your content and improve patient compliance. We've put together examples of how motion graphics can be used effectively in digital therapeutics.







